Generation of optimally-sized primary human pancreatic microtissues through single cell dissociation and a novel suspension microcavity system is associated with resolution of disrupted organelle ultrastructure and reestablishment of cell-cell interactions
Morgan Shaw1, Nicola J Dyson1, Minna Honkanen-Scott1, Markus Muhlemann4, Nicole Kattner1, Rowen Coulthard3, Catherine Arden2, James A M Shaw1, Patrick Kugelmeier4, William E Scott III1.
1Translational and Clinical Research Institute, Newcastle University, Newcastle, United Kingdom; 2Biosciences Institute, Newcastle University, Newcastle, United Kingdom; 3Department of Cellular Pathology, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, United Kingdom; 4Kugelmeiers Ltd., Erlenbach, Switzerland
Introduction: Acute endocrine cell stress in donor pancreas due to hypoxia and islets >150 µm diameter post-isolation are associated with impaired clinical transplant outcomes1. Cell-cell interactions enhance islet beta-cell function. We aimed to evaluate the use of Kugelmeiers SphericalPlate 5D (SP5D; Kugelmeiers Ltd., Switzerland), a novel 3D microcavity suspension-well system for generation and maintenance of optimally-sized viable engineered islet microtissues and to assess impact on endocrine acute stress and cell-cell interactions.
Methods: Primary human islets were isolated using the semi-automated Ricordi method. A tissue biopsy from the head region was taken pre-isolation and isolated islets were sampled 1 hr following isolation (D0). Islets were dissociated into single cell suspensions through addition of Accutase (Sigma-Aldrich, UK) and mechanical agitation in a 37 °C water bath. Cells were seeded at densities of 187,500, 375,000 and 562,500 cells/well into SP5D and maintained for 120 hr. Paired control islets were maintained for 120 hr in non-adherent T75 flasks. Tissues were maintained at 37 °C in 5% CO2 with SP5D and control organoid diameter visually assessed (graticule) and viability estimated following propidium iodide staining (n=50 engineered islet microtissues / control islets). Samples from pre-isolation biopsy, D0 islets and 120 hr islet microtissues and islets were processed for transmission electron microscopy and imaged following established protocols, with endocrine cells (25 per sample) assessed using the Newcastle Pancreas Endocrine Stress Score (NPESS)2.
Results: Following 120 hr preservation, mean engineered islet microtissue diameter of ⁓100 µm was observed in SP5D with none >150 µm (seeding at 562,500 cells/well equivalent to 750 cells/microwell yielded diameter 101.8±7.7 µm). For control islets, mean diameter was significantly higher (132.2±63.1 µm; p=0.001) and less consistent with islets of 300 µm diameter observed. Viability following 120 hr preservation was higher for SP5D (89±8%) vs control (70±22%; p<0.0001). Total NPESS score was improved for SP5D (5.6) vs biopsy (8.4) and D0 (6.5) and was comparable to control (5.22). SP5D had the lowest score for mitochondrial swelling (1.2) vs biopsy (2.7), D0 (1.9), and control (1.2); and cytoplasmic vacuolisation SP5D (0.8) vs biopsy (1.0), D0 (1.1), and control (0.8). Ultrastructural cell-cell interactions were observed in SP5D.
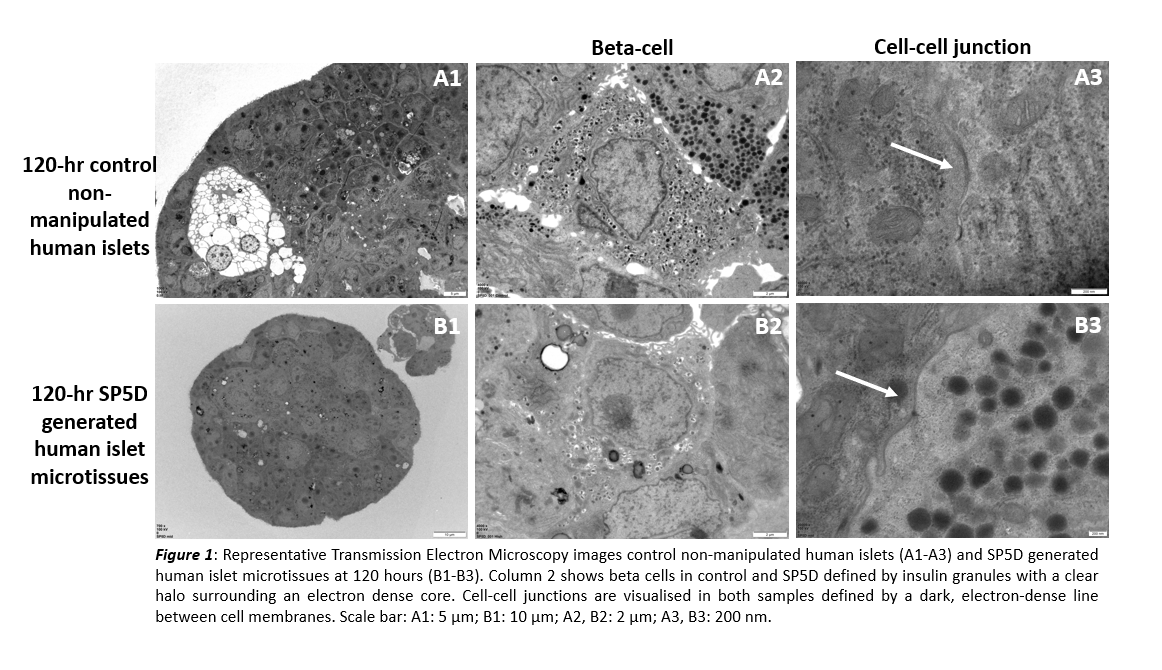
Conclusion: We have developed and validated standardised protocols for preparing viable islet microtissues from primary human pancreatic cells using SP5D. Optimal and consistent diameter size of ~100 µm was attained. SP5D demonstrated improved total NPESS score compared to biopsy and D0 islets, with lowest score for mitochondrial swelling and cytoplasmic vacuolisation compared to all other samples. Cell-cell interactions in SP5D further support formation of microtissues mimicking native islets.
National Institute for Health and Care Research (NIHR) Blood and Transplant Research Unit in Organ Donation and Transplantation (NIHR203332).
[1] 1 Lehmann R, Zuellig RA, Kugelmeier P, Baenninger PB, Moritz W, Perren A, Clavien PA, Weber M, Spinas GA. Superiority of small islets in human islet transplantation. Diabetes. 2007 Mar;56(3):594-603 2807/3000
[2] 2 Dyson NJ, Kattner N, Honkanen-Scott M, Hunter B, Doyle JA, White K, Davey TS, Ploeg RJ, Bury YA, Tiniakos DG, Shaw JAM, Scott WE 3rd. Development and Application of a Semi quantitative Scoring Method for Ultrastructural Assessment of Acute Stress in Pancreatic Islets. Transplant Direct. 2021 Dec 16;8(1):e1271.