A conceptual supportive cell based implant for beta cell replacement therapy
Carolin Hermanns1, Aylin Seedorf1, Timo Rademakers1, Aart van Apeldoorn1.
1MERLN Institute for Technology-Inspired Regenerative Medicine, Maastricht University, Maastricht, Netherlands
Background: Currently, the consensus in the field is that beta cell delivery devices are the go-to strategy for beta cell replacement therapy. They provide a safe and controlled way of implanting allogeneic cells, can protect, and be tuned to support beta cell function and survival. However, they are predominantly based on synthetic biomaterials which can elicit oxidative or inflammatory cell stress. A number of studies have shown that addition of support cells or extracellular matrix (ECM) can improve survival and function of beta cells. Mesenchymal stem cells have been shown to improve cell survival and function, while specific ECM molecules can support beta cell function, and endothelial cells improve vascularization. The logical next step would be to bioengineer an entirely cell-based delivery strategy to overcome some of the disadvantages of synthetic delivery devices. Here we report on a conceptual cell based delivery strategy aiming to create a more natural environment for islets and beta cells using a combination of different layers of supportive cell types namely MSCs and endothelial cells stacked with islets inbetween.This cell based delivery strategy could be tailored to be patient specific and can be combined with stem cell derived beta cells.
Methods: NUNC UpCell dishes were used to create cell sheets from immortalized MSCs. Several cell sheets were stacked on top of each other to encapsulate human donor islets in-between in a sandwich like construct. Prevascularization was done by co-culturing human umbilical vein endothelial cells and iMSCs in separate cells sheets which can be added to the sandwich construct. We studied the distribution of the different cells, beta cell aggregates and islets, using confocal fluorescence microscopy by a combination of GFP expressing beta cells and immunofluorescence, and beta cell function using GSIS.
Results: Optimization of culture protocols of cell sheets lead to stable and easy to handle iMSC sheets. Stacking of multiple cell sheets further improved the stability and handling of the multilayered cell-based construct in which islets and beta cell aggregates can be retained (Figure 1). The embedded islets were able to secrete insulin in response to glucose once embedded inside the cell construct. Furthermore, co-culture of HUVECs and iMSCs lead to a dense vascular like network within cell sheets (Figure 2).
" href="https://cm.sandiego2023.org/papers/body/199#">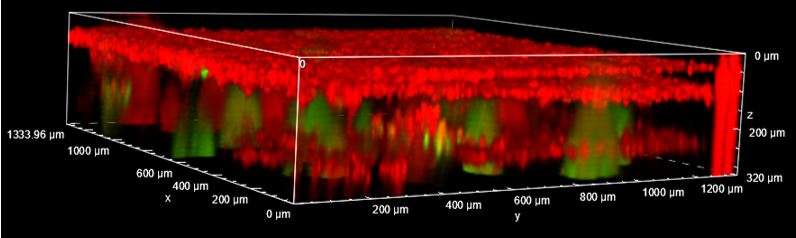
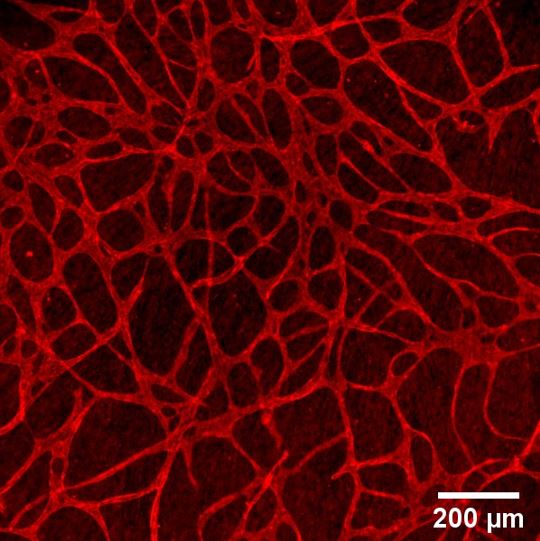
Conclusion: Individual iMSC sheets can be stacked into a multilayered sandwich like construct with human donor islets in between. The human islets inside these multilayered cell constructs show a proper glucose response. The addition of HUVECs leads to a vascular like network in these cell sheets. Our next goal is upscaling this technology to perform small animal studies and study its clinical translatability.