Functional subcutaneous engraftment of human islets achieved by co-transplantation with vasculogenic and adaptable endothelial cells
Ge Li1,2, Rebecca Craig-Schapiro1, David Redmond1, Kevin Chen1, Shahin Rafii1.
1Medicine, Weill Cornell Medical College, New York, NY, United States; 2Bronx Community College, City University of New York, New York, NY, United States
Introduction: The subcutaneous space is an attractive site for islet transplantation due to the ease of accessing, monitoring, and retrieval[1, 2]. However, achieving functional engraftment of subcutaneously transplanted islets remains a challenge, and insufficient vascularization is a major hurdle[3]. We have reported that transiently overexpressing ETV-2 (a pioneer transcription factor during vascular development) converted generic adult endothelial cells (ECs) to Re-programmed Vascular ECs (R-VECs), which self-assembled in microfluidic devices to form perfusable tubes that carried human blood and established a long-lasting vascular network within the subcutaneous space after mouse transplantation[4]. Hence, we hypothesized that co-transplantation of R-VECs would enable the functional engraftment of subcutaneously transplanted human islets.
Method: To test it, 3,000 IEQ human islets and 1 million R-VECs in 200 μL collagen hydrogel were subcutaneously transplanted into streptozotocin-induced diabetic SCID-beige mice (n=21). As controls 4 mice received human islets alone and 5 mice received human islets and generic ECs derived from human umbilical cord vein (HUVECs).
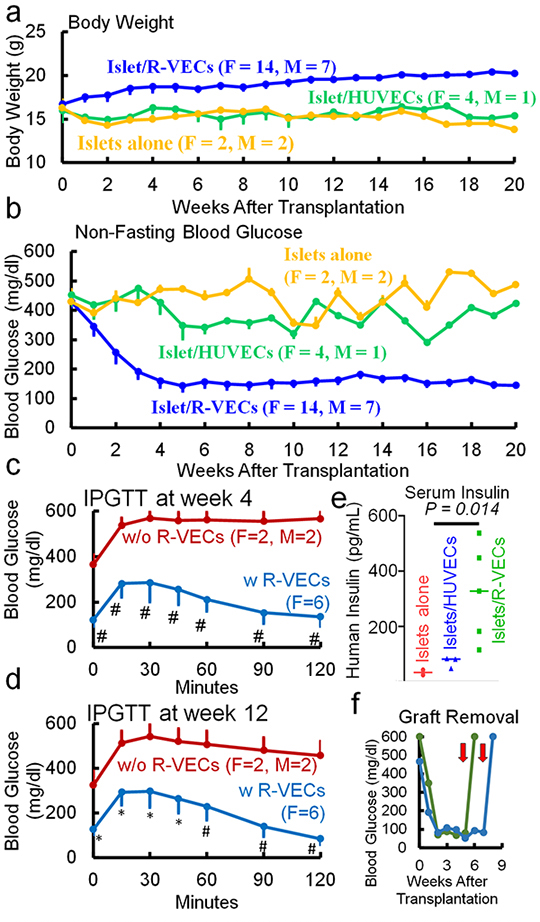
Results: Only islet/R-VEC, but not islet alone or islet/HUVEC, transplantation resulted in body weight stabilization and hyperglycemia reversal in the diabetic mice (Fig. 1a,b). In addition, islet/R-VEC-transplanted mice showed improved glucose clearance rates (Fig. 1c,d) and high levels of serum human insulin (Fig. 1e). The efficacy of the islet/R-VEC subcutaneous transplantation was further confirmed by removal of the subcutaneous grafts, which resulted in hyperglycemia (Fig. 1f).
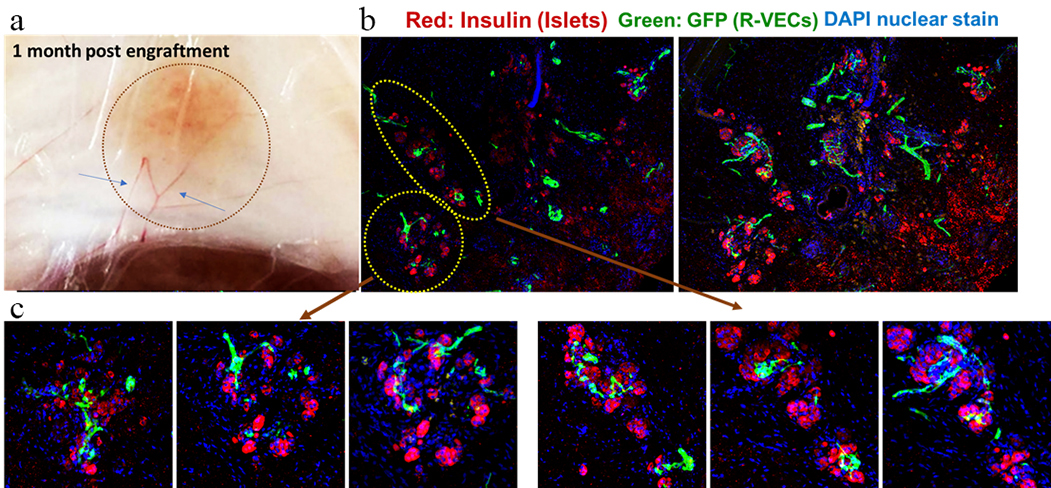
Explanted grafts were richly vascularized, with multiple large vessels connected to the graft (Fig. 2a). Islets sustained architectural integrity (Fig. 2b), and continuous sections showed blood vessel penetration inside the islets (Fig. 2c), thus reflecting the structure of native pancreatic islets.
Conclusion: R-VECs enabled the functional engraftment of subcutaneously transplanted islets and restored euglycemia in diabetic mice, which was mediated by enhanced vascularization and the establishment of the local vascular niche mimicking that of native islets in the pancreas.
JDRF. Russell Berrie Foundation.
[1] 1. Aghazadeh, Y., et al., Microvessels support engraftment and functionality of human islets and hESC-derived pancreatic progenitors in diabetes models. Cell Stem Cell, 2021. 28(11): p. 1936-1949 e8.
[2] 2. Yu, M., et al., Islet transplantation in the subcutaneous space achieves long-term euglycaemia in preclinical models of type 1 diabetes. Nat Metab, 2020. 2(10): p. 1013-1020.
[3] 3. Ramzy, A., et al., Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell, 2021. 28(12): p. 2047-2061 e5.
[4] 4. Palikuqi, B., et al., Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature, 2020. 585(7825): p. 426-432.