Preserving Liver Natural Killer Cell Activity: Developing Novel Culture Techniques for "Off-the-Shelf" Cell Immunotherapy
Yuki Imaoka1,2, Miho Akabane1, Masahiro Ohira2, Hideki Ohdan2, Kazunari Sasaki1.
1Division of Abdominal Transplant, Stanford University, Stanford, CA, United States; 2Gastroenterological and Transplant Surgery, Hiroshima University, Hiroshima, Japan
Introduction: Natural killer (NK) cells are cytotoxic lymphocytes that belong to the innate immune system and are capable of selectively targeting and eliminating virally infected and cancerous cells. As our knowledge of the molecular mechanisms governing NK cell activity expands, their potential in cancer immunotherapy becomes increasingly promising. Despite the strict selection criteria, the recurrence rate of hepatocellular carcinoma (HCC) after liver transplantation (LTx) remains high at 15-20%. While adoptive immunotherapy using liver-derived NK cells for LTx with HCC shows promise as a treatment option, personalized immunotherapy is considered ideal.
The development of culture methodologies following thawing is critical for universal accessibility to NK cell therapy, regardless of time and location. However, the expression of activating receptors, such as TNF-related apoptosis-inducing ligand (TRAIL) and NKG2D on liver NK cells is diminished after exposure to liquid nitrogen, even after IL-2 stimulation. This study aims to develop culture techniques for preserved NK cells focusing on PI3K/AKT/mTOR signaling, to create an "off-the-shelf" liver-derived NK cell therapy, for wider availability.
Methods: The study enrolled liver NK cells from 7 deceased donors (DD), and peripheral NK cells from 5 healthy volunteers (HV). Lymphocytes were cultured with human recombinant IL-2 (as a control) and IL-33 or IL-37 (which inhibits IL-33 signaling) for three days. The IL-33 pathway promotes the maturation of liver NK cells, leading to reduced expression of TRAIL and decreased anti-tumor cytotoxicity via PI3K/AKT/mTOR signaling in mice models.
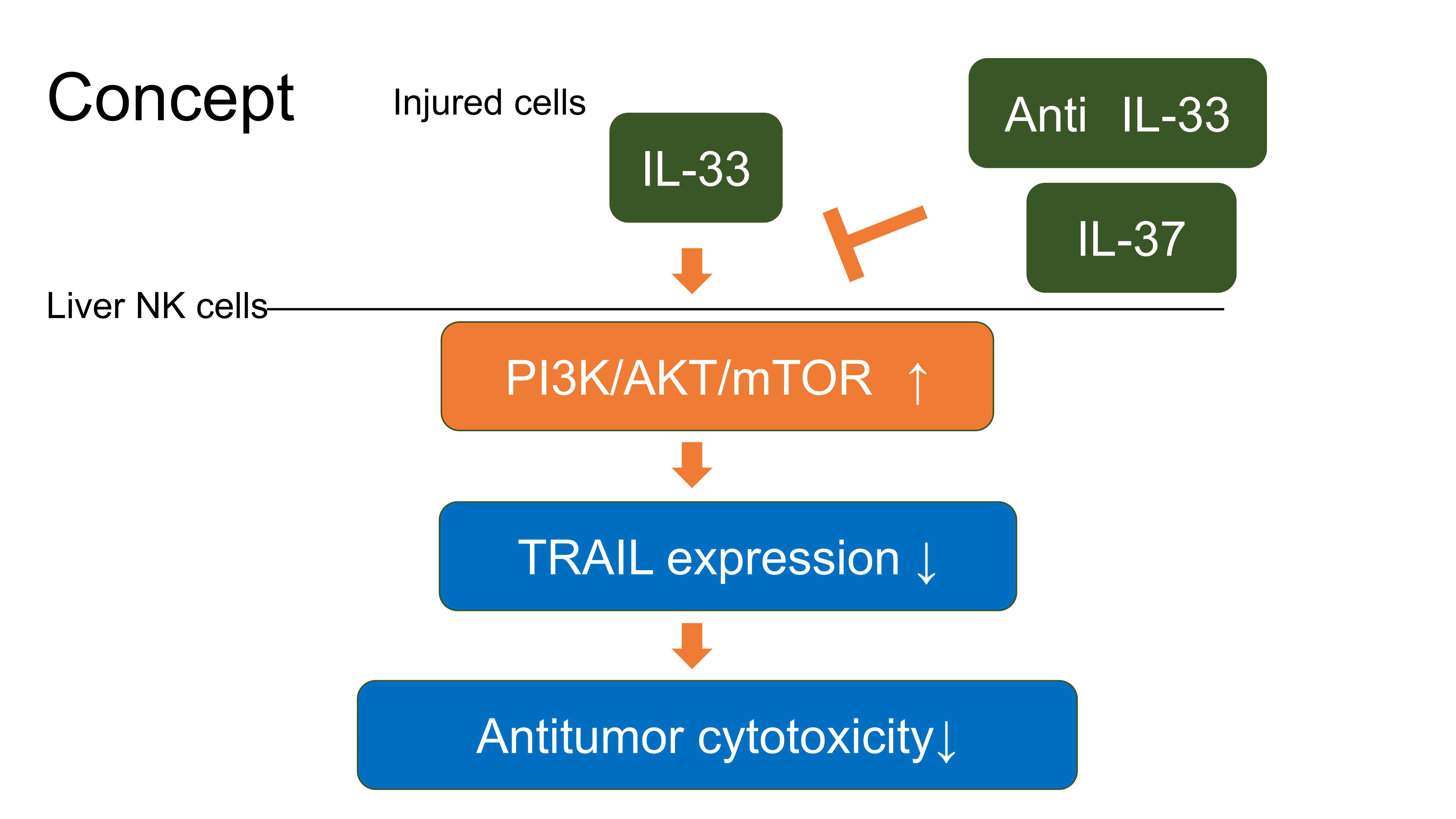
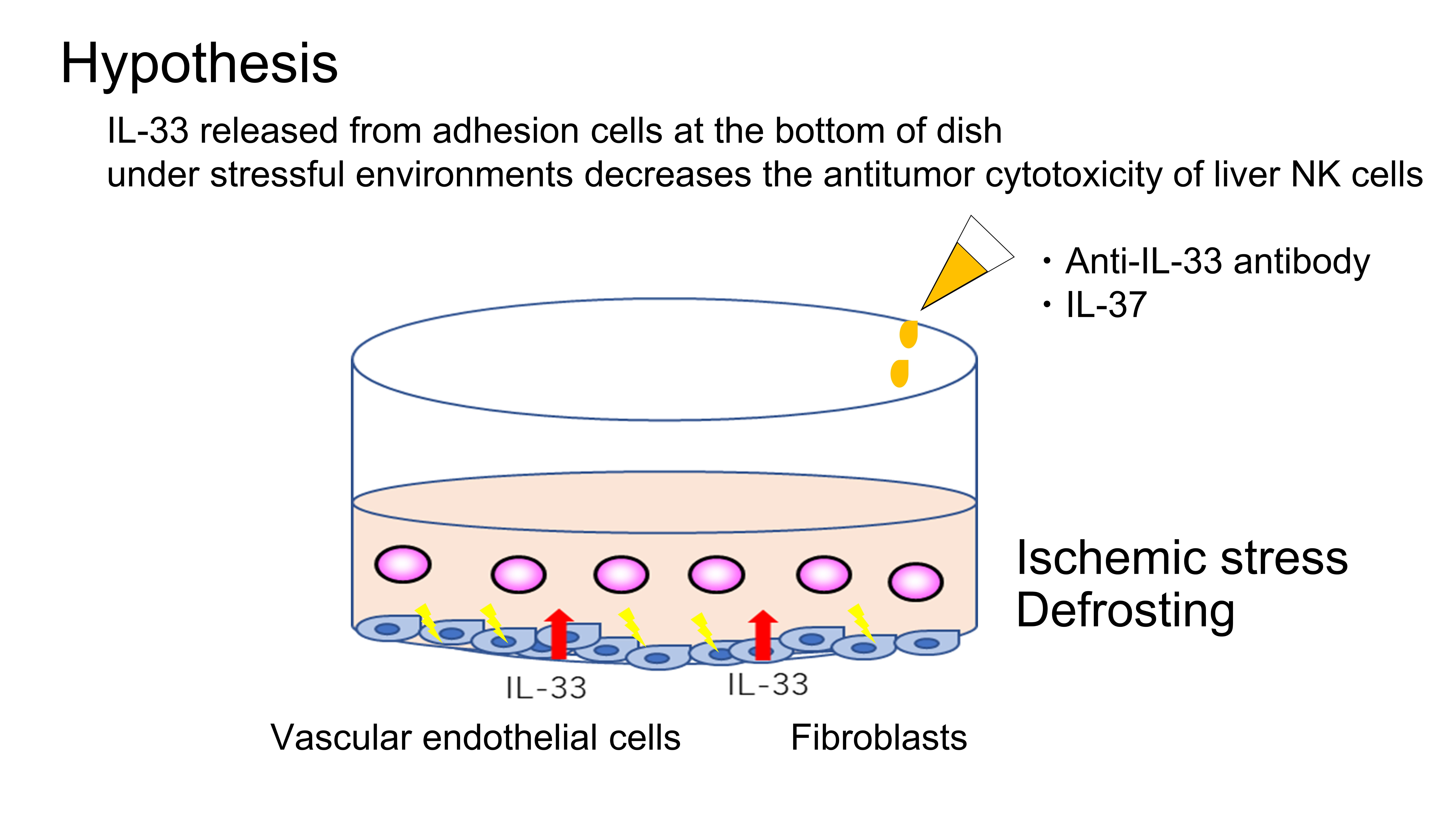
Our hypothesis is that under stressful conditions such as freezing or ischemia, IL-33 is released from adhesion cells at the bottom of the dish.
Results
1) In fresh peripheral NK cells (N=5), TRAIL (29.1% vs 18.5%), NKp44 (25.1% vs 20.0%) and NKp46 (84.8% vs 76.4%) expression on NK cells decreased by 35%, 20% and 10% respectively after IL-33 stimulation.
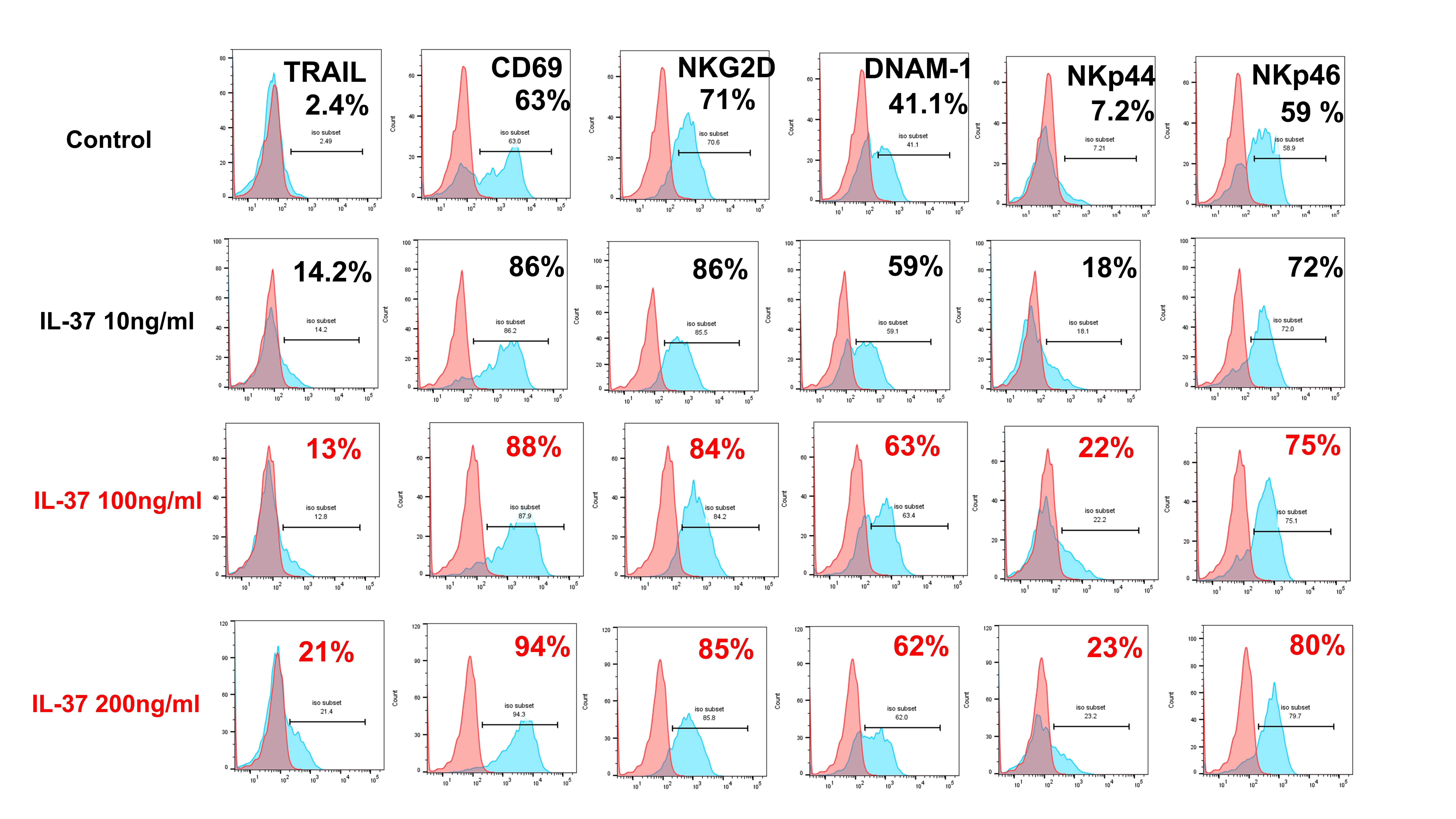
2) In fresh liver NK cells (N=7), TRAIL (31.3% vs 20.1%) and NKp44 (30.5% vs 18.9%) expression on NK cells decreased by 35.6% and 28.1%, respectively after IL-33 stimulation. TRAIL (17.9% vs 24.3%) and NKp44 (22.8% vs 28.2%) expression on NK cells increased by 35.8%, and 23.7%, respectively after IL-37 stimulation.
3) After defrosting, liver NK cells (N=2), the activation receptors were improving, depending on the concentration of IL-37 levels.
Conclusion: Inhibition of IL-33 signaling has the potential to preserve liver NK cell activity even after exposure to liquid nitrogen. The development of novel culture techniques post-thawing is crucial for enabling universal access to liver NK cell therapy, independent of temporal and spatial restrictions, and for establishing "off-the-shelf" liver NK cell therapy following LTx for HCC.