Can pyelonephritis initiate antibody-mediated rejection (AMR) of a pig xenograft?
Kohei Kinoshita1, Akihiro Maenaka1, Ivy A Rosales1,2, Tatsuo Kawai1, David Ayares4, Yuko Miwa3, Takaaki Kobayashi3, David K Cooper1.
1Center for Transplantation Sciences, Department of Surgery, Massachusetts General Hospital/Harvard Medical School, Boston, MA, United States; 2Department of Pathology, Immunopathology Research Laboratory, Massachusetts General Hospital/Harvard Medical School, Boston, MA, United States; 3Aichi Medical University, Nagakute, Japan; 4Revivicor, Inc, Blacksburg, VA, United States
Introduction: AMR of an ABO-incompatible kidney allograft initiated by pyelonephritis is well-recognized. We summarize one such case and provide data on a baboon with a pig kidney graft in which we suspect AMR was initiated by pyelonephritis.
Methods and Results: After desensitization, a blood type B patient received a type A kidney allograft. Therapy was with basiliximab, cyclosporine, MMF, and steroids. A Foley catheter was placed. Initial kidney function was good, but on POD8, E.coli was detected in the urine (from POD6), and reduction in diastolic graft blood flow and increase in serum creatinine were observed. From POD8-14, both anti-rejection therapy, including steroids, plasmapheresis, rituximab, IVIg and recombinant thrombomodulin, and antibiotic therapy were conducted. Despite this treatment, the graft did not recover function, and was removed on POD20. Histopathology of graft biopsy on POD15 confirmed thrombotic microangiopathy probably due to AMR. Retrospective studies showed rapid increases in not only anti-A, but also anti-B and anti-Gal antibodies.
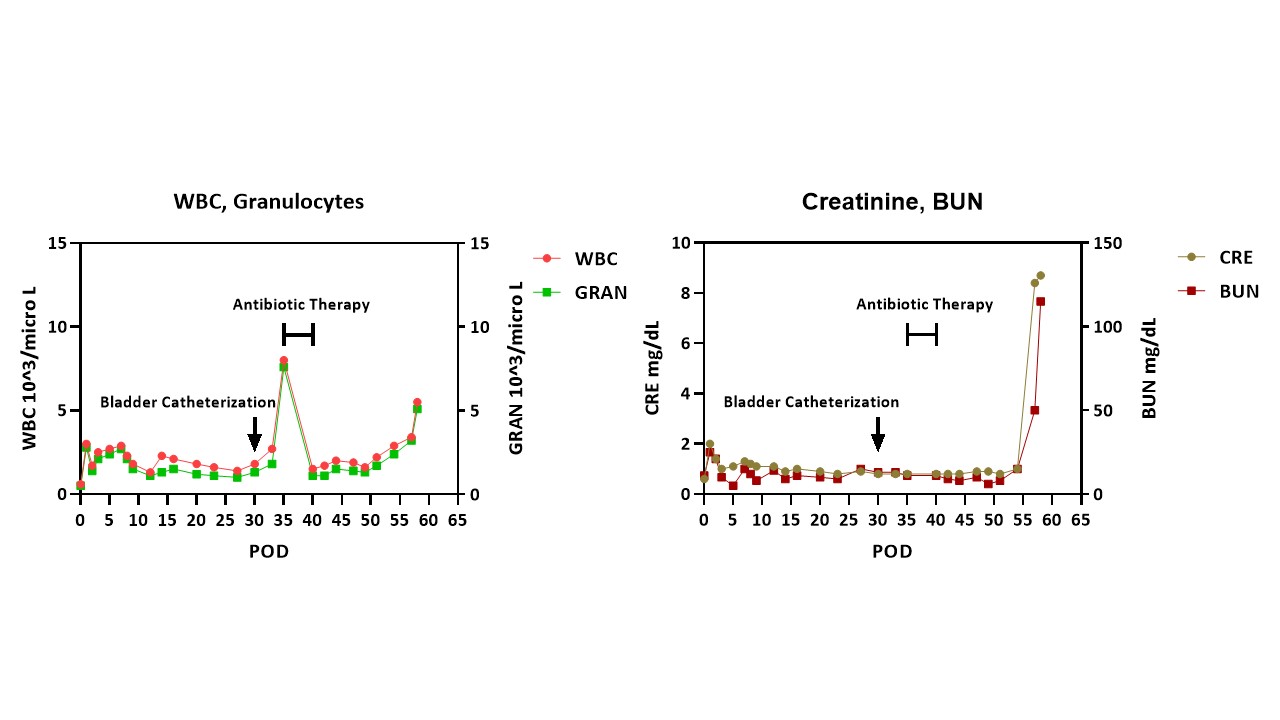
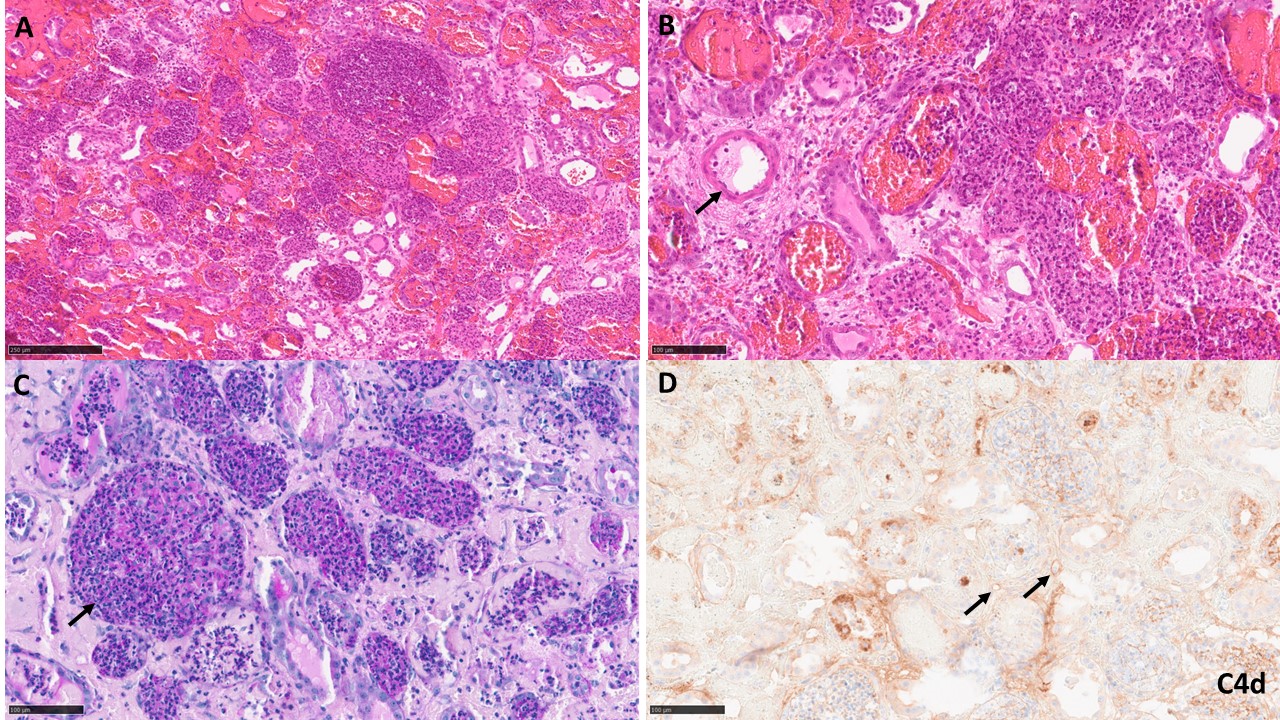
A baboon (blood type B) received a kidney from a pig (type O) with 10 gene edits. Induction therapy was with ATG, rituximab, and a C1-esterase inhibitor, and maintenance with an anti-CD154mAb, rapamycin, steroids, and tocilizumab. Pig kidney function was excellent for almost 2 months (Figure1 right). However, a catheter was passed on day 30 to obtain urine for measurement of proteinuria. Within 4 days, an increase in the granulocyte count was detected (Figure1 left). Antibiotic therapy was initiated, resulting in a temporary reduction in white blood count before it began to increase again. Urine culture revealed no microorganisms. However, the creatinine rapidly rose from 1.0 to 8.4mg/dl, necessitating euthanasia. Histopathology showed unusual neutrophil infiltrates suggestive of pyelonephritis (and/or possibly AMR) (Figure2), Retrospective measurement of serum showed no increase in anti-TKO pig antibodies.
Conclusions: We suggest that the E.coli infection initiated AMR in the kidney allograft. It is speculated that E.coli express some common carbohydrates for blood type A, B and Gal antigens. In the pig-to-baboon model, we suggest that the increase in granulocytes after bladder catheterization resulted from a urinary infection, but no microorganism was identified because antibiotics had been administered. The rapid rise in serum creatinine in a previously quiescent graft (relative absence of proteinuria) and the unusual neutrophil infiltrates in the graft suggest that pyelonephritis may have been an initiating factor in AMR. The lack of increase in anti-TKO antibodies may have been because of the short duration between activation of the immune system and euthanasia, all antibodies being bound to the graft. Investigation, e.g., ELISA, is continuing. Although the evidence is limited in this case, if a urological infection can initiate AMR, this could be problematic after xenotransplantation.