
Beneficial effects of microvascular fragments and stromal vascular fraction cells collected from human peripancreatic fat for stimulating intra-islet endothelial cells to form peri-islet vessels in human islets during co-culture and co-transplantation
Ahad A. Kodipad1, Krishna K. Samaga1, Chandrashekar B. Revanna,1, Siddharth Narayanan1, Jaimie D Nathan1, Appakalai N. Balamurugan1.
1Pediatrics and Surgery, Center for Clinical and Translational Research, Nationwide Children's Hospital / Ohio State University, Columbus, OH, United States
Transplanted islets undergo apoptosis immediately after transplantation due to poor blood supply especially the inner core cells. Islet isolation process severs existing islet vascular connections. As such, these islets become avascular and are susceptible to ischemic damage eventually resulting in metabolic exhaustion and islet dysfunction. In vitro stimulation of intra-islet endothelial cells to form peri-islet vessels (PIV’s) prior to transplantation may accelerate post-implant islet revascularization. Adipose-derived stromal vascular fraction cells (SVF) are known to create microvascular networks and release angiogenic tropic factors. We have evaluated for the first time, the effect of peripancreatic fat-derived microvascular fragments (MVF) and stromal vascular fraction (PPSVF) cells on PIV formation in a 3D-culture system and in vivo model.
Human islets were isolated from brain-dead donor pancreases (n=18) according to our laboratory protocol. Peripancreatic fat was collected around the pancreas and PPSVF cells were isolated using the MVF and SVF cell isolation methods. Complete characterization was done for these two types of cell fractions. Isolated human islets were cultured in a 3D collagen gel (rat tail collagen-1) for 14 days with MVF’s or PPSVF cells (250,000 cells). Confocal microscopy was used to assess islet-derived PIV growth and MVF and PPSVF cell-vascular networks. Time-lapse microscopy (Cytation 5) was used to monitor islet-derived PIV growth every day for 14 days. After the sprout formation, human endothelium was identified via labeling with UEA-1 staining. To study diabetes reversal, the islets were implanted into diabetic nude mice at the subcutaneous site.
MVF and PPSVF cells contained predominantly pancreatic endothelial cells. Human islets co-cultured with MVF’s or PPSVF cells induced cellular sprouting within 7 days. Many of these sprouts appeared to be PIV since they resembled a vasculature that arose from the islets.
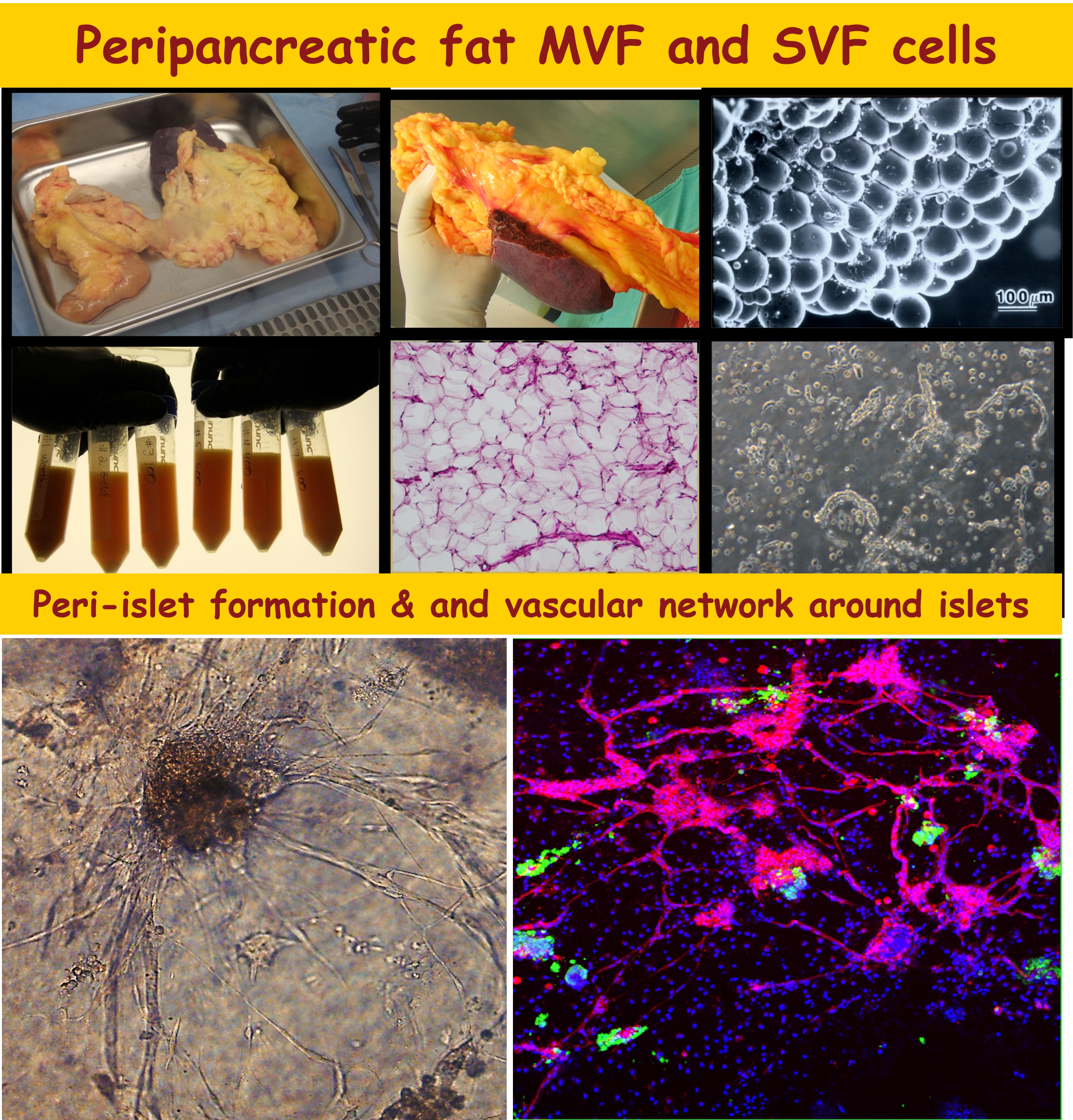
Our labeling indicated that several of these sprouts were endothelial in origin. Intra-islet endothelial cells were stained with UEA-1 3D-cultured islets maintained >90% cellular viability after 14 days, whereas 2D-cultured islets displayed disintegration of their borders as well as reduced viability. Statistically significant difference (P<0.05) was observed in number of sprouts when islets were cultured in 3D environment without MVF or PPSVF cells. Quick reversal of diabetes was observed after co-transplantation of islet+MVF and Islet+PPSVF cells diabetic nude mice.
We have successfully utilized peripancreatic MVF and SVF cells for co-culture and co-transplantation studies. We have also successfully demonstrated the formation of peri-islet vessels from human islets with MVF’s and PPSVF cells, in a 3D matrix support. MVF and PPSVF within a 3D culture may provide unique avenues to accelerate islet neovascularization following transplantation.